Ph Value of Ethanoic Acid
Ethanoic acid CH 3 COOH belongs to the group of carboxylic acids and is commonly called as acetic acid. The molecular and structural formula of.
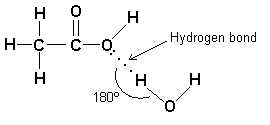
Solubility And Ph Of Ethanoic Acid
These pH values are lower than the pKa value of.

. Acetic acid is produced by. It is slightly heavier than water with a density of 105 gcm 3. When all ethanoic acid is finished when reaction is completed pH value will be just above than 7 because sodium ethanoate is a weak base.
Because H 3 O concentration is known now pH value of acetic. Now as long as the initial concentration of the acetic acid c is significantly higher than the Ksp of the acid you can use the approximation. The results are listed in the following tables valid for.
65 rows Based on given acidity constants pK a values the pH of organic acids for 1 10 and 100 mmolL are calculated. To ask about a 6 aqueous solution of ethanoic acid has no meaning. After completion of the reaction if you add one.
C x. There are 3 bottles which. You will get a different answer depending on which you.
Ethanol is basic in nature. These pH values are lower than the pKa value of. Ksp x x c x x2 c x.
We can see a relationship between pH value when we dilute the acid by 10 100 1000 times. Adams in Encyclopedia of Food Microbiology Second Edition 2014 Introduction. Viva Questions on To find the pH of the dilute hydrochloric acid dilute NaOH solution dilute ethanoic acid solution lemon juice water and dilute sodium hydrogen carbonate solution.
The pH value of the feed phases of 01 M 005 M and 001 M concentrations of acetic acid was found to be 323 365 and 405 respectively. Answer 1 of 2. H 3 O is given by water is neglected because dissociation of water is very low compared to the acetic acid dissociation.
The pH value for Ethanoic acid is 3. Acidity constants are taken from here. The pH value of ethanol is 733.
The pH value of the feed phases of 01 M 005 M and 001 M concentrations of acetic acid was found to be 323 365 and 405 respectively. Is it 6 vv 6vm 6 mv or 6 mm. PH values of different concentrations of ethanoic acid solutions.
Calculated pH values of common acids and bases for 1 10 and 100 mmolL valid for standard conditions at 25 1 atm. After adding 5-8 of. Vinegar is essentially a dilute solution of acetic ethanoic acid in water.
Ethanoic acids pure state is acidic.

To Find The Ph Of The Samples By Using Ph Paper Universal Indicator Lab Work

Ph O Level Secondary Chemistry Tuition

What Is Ethanoic Acid The Chemistry Blog
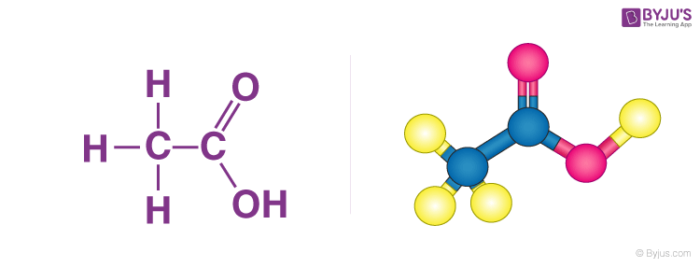
Ethanoic Acid Properties Structure Uses Reactions And Faqs Of Ethanoic Acid
Comments
Post a Comment